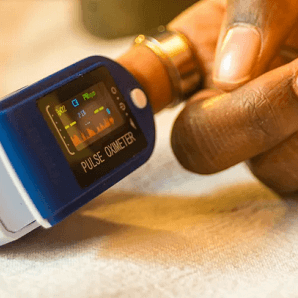
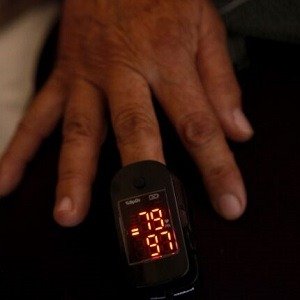
A Food and Drug Administration (FDA) panel has recommended increased diversity in pulse oximeter trials, following revelations of racial and ethnic disparities in the device’s readings.
The panel’s recommendation comes in the wake of a 2022 study that found Asian, Black, and Hispanic patients in intensive care received less supplemental oxygen than their White counterparts, a discrepancy linked to differences in pulse oximeter readings.
The study challenged the prevailing assumption that health disparities were primarily due to social determinants such as environmental racism, lack of access to care, and lower employment status. While these factors undeniably impact health outcomes in Black and brown populations, the pandemic has prompted the medical community to delve deeper into the causes of disparity in outcomes, including potential bias in medical devices like the pulse oximeter.
See “FDA panel recommends more diversity in pulse oximeter trials” by Jacqueline Howard on the CNN website (February 2, 2024)