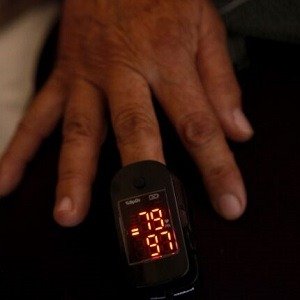
he U.S. Food and Drug Administration has released draft guidance aimed at improving the performance of pulse oximeters across diverse skin tones. This move addresses longstanding concerns about the devices’ accuracy in measuring blood oxygen levels in individuals with darker skin pigmentation.
Pulse oximeters, which estimate blood oxygen saturation by clipping onto a fingertip, have been widely used in medical settings and gained popularity during the COVID-19 pandemic. However, research has consistently shown that these devices can overestimate oxygen levels in people with darker skin, potentially leading to delayed or missed treatment.
The FDA’s proposed recommendations include increasing the diversity of participants in clinical studies, with at least 25% of subjects falling within each skin color group on the Monk Skin Tone scale. Manufacturers are also advised to use both subjective and objective methods to evaluate skin tone and assess device performance across skin pigmentations.
Dr. Michelle Tarver, director of the FDA’s Center for Devices and Radiological Health, emphasized that the draft guidance is “based on the best available science to help address concerns of disparate performance of pulse oximeters based on an individual’s skin pigmentation.”
The agency is also considering creating a public webpage to identify FDA-cleared pulse oximeters that demonstrate comparable performance across skin pigmentations. This move aims to help healthcare providers and consumers make informed decisions about device selection.
While some currently marketed pulse oximeters may already meet the updated performance criteria, the FDA expects that implementing these recommendations will significantly improve the accuracy and reliability of these crucial medical devices for all patients, regardless of skin color.
See: “FDA Proposes Updated Recommendations to Help Improve Performance of Pulse Oximeters Across Skin Tones” (January 06, 2025)